hcooch ch2 h2o reaction explained: From ester to essential acids
Esters are essential compounds in organic chemistry and industry, offering a wide range of applications from solvents to fuel additives. Among the numerous ester reactions studied, the hydrolysis of methyl formate is particularly important. This article explores the reaction commonly represented by the keyword hcooch ch2 h2o, providing a detailed understanding of the chemical process, its mechanism, significance, and real-world uses. Whether you’re a chemistry enthusiast or a student, this comprehensive explanation of the hcooch ch2 h2o reaction offers valuable insights into a critical aspect of chemical engineering.
What is hcooch ch2 h2o and what does it represent
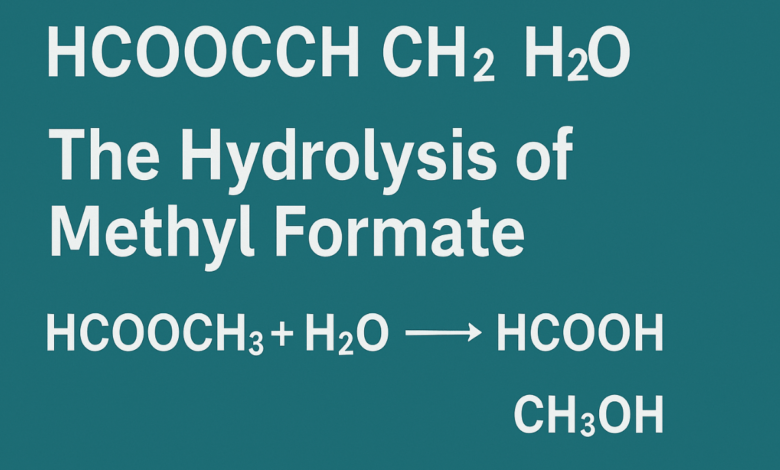
The keyword hcooch ch2 h2o is not a standard chemical formula but appears to be a shorthand or typographical variant that refers to the reaction of methyl formate with water. In chemical terms, this reaction can be described as:
HCOOCH3 + H2O → HCOOH + CH3OH
This hydrolysis reaction results in the production of formic acid and methanol. It is a well-known process in organic chemistry and is widely applied in industrial environments for the efficient synthesis of useful chemicals.
The keyword hcooch ch2 h2o likely symbolizes the elements and compounds involved in this reaction, capturing the essential reactants and outcomes of the process.
Introduction to methyl formate and its significance
Methyl formate, chemically expressed as HCOOCH3, is a simple ester formed from formic acid and methanol. It is a colorless, flammable liquid with a pleasant odor, and it plays a key role in the manufacture of various chemicals. In industries, methyl formate is used as a blowing agent, solvent, and as an intermediate for the production of formic acid and other derivatives.
The reaction of hcooch ch2 h2o involves methyl formate reacting with water to form useful products. Understanding this process is crucial for optimizing industrial output and maintaining chemical efficiency in manufacturing environments.
How the hydrolysis reaction works
The hcooch ch2 h2o reaction takes place through a process known as ester hydrolysis. This mechanism involves breaking the ester bond in the presence of water. Under acidic conditions, the hydrolysis reaction proceeds through the following steps:
- Protonation of the ester’s carbonyl oxygen increases its reactivity.
- A water molecule attacks the carbonyl carbon, forming a tetrahedral intermediate.
- Proton transfers lead to the departure of the alcohol group.
- The final products are formic acid (HCOOH) and methanol (CH3OH).
This reaction is reversible and can be influenced by various external conditions such as temperature, water availability, and catalysts. The representation hcooch ch2 h2o serves as a simplified expression of this important chemical transformation.
Industrial applications of hcooch ch2 h2o
The reaction denoted by hcooch ch2 h2o is not just of academic interest but has important industrial applications. Two valuable products are obtained through this reaction:
- Formic acid: This acid is widely used in leather tanning, rubber production, dyeing textiles, and as a preservative in animal feed. It is also used as a coagulant in natural rubber production.
- Methanol: Known as wood alcohol, methanol is used in the production of formaldehyde, antifreeze, fuels, and various chemical compounds.
By utilizing the hcooch ch2 h2o reaction, industries are able to synthesize both formic acid and methanol simultaneously, making the process cost-effective and efficient.
Factors affecting the reaction efficiency
The hydrolysis process represented by hcooch ch2 h2o can be influenced by several external factors. Understanding these conditions is essential for controlling the reaction and optimizing yield.
- Catalysts: The reaction is typically carried out in the presence of an acid catalyst such as sulfuric acid, which helps speed up the breakdown of the ester.
- Temperature: Increasing the temperature can accelerate the reaction rate but must be carefully monitored to avoid unwanted side reactions.
- Water concentration: An excess of water helps drive the equilibrium toward the formation of products, improving overall efficiency.
- Reaction medium: Acidic environments are preferred for better conversion rates and simpler product separation.
Each of these factors plays a key role in determining the outcome of the hcooch ch2 h2o reaction in both lab and industrial settings.
Safety considerations when handling the chemicals
While the hcooch ch2 h2o reaction offers practical benefits, it is essential to understand the safety aspects of working with the involved chemicals.
- Methyl formate is flammable and can be harmful if inhaled in large quantities.
- Formic acid is corrosive and can cause skin burns upon contact.
- Methanol is highly toxic and poses health risks if ingested or absorbed through the skin.
Proper storage, ventilation, and the use of personal protective equipment are crucial when handling these substances. This ensures safety for workers and minimizes the risk of industrial accidents.
Environmental and economic relevance of hcooch ch2 h2o
The hcooch ch2 h2o reaction offers environmental benefits when managed correctly. The by-products are biodegradable and less harmful than other synthetic chemicals. Additionally, the process allows industries to make dual-use of raw materials, thereby reducing waste and improving cost-efficiency.
Methanol can also serve as an alternative fuel source, contributing to greener energy solutions. As environmental regulations become stricter, processes like hcooch ch2 h2o offer sustainable pathways for chemical production.
Conclusion
The reaction represented by hcooch ch2 h2o plays a vital role in the world of chemistry and industry. From the efficient production of formic acid and methanol to its implications in sustainable manufacturing, this process is a cornerstone of modern chemical engineering. Understanding the underlying mechanisms and practical applications of hcooch ch2 h2o provides valuable insights for students, professionals, and industry experts alike. As technology evolves, mastering such foundational reactions will remain essential for innovation and efficiency in the chemical sector.
Frequently Asked Questions (FAQs)
1. What does hcooch ch2 h2o refer to in chemistry?
It refers to the hydrolysis of methyl formate with water, producing formic acid and methanol.
2. What are the main products of the hcooch ch2 h2o reaction?
The main products are formic acid and methanol.
3. Why is the hcooch ch2 h2o reaction important in industry?
It allows for the simultaneous production of two valuable chemicals used in multiple industrial applications.
4. What conditions are required for the hcooch ch2 h2o reaction to occur efficiently?
Acidic catalysts, sufficient water supply, and elevated temperatures help optimize the reaction.
5. Are there safety concerns with the hcooch ch2 h2o reaction?
Yes, proper precautions are needed as the chemicals involved can be flammable, toxic, or corrosive.



